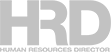
These rapid tests signal a revolutionary turn in the fight against COVID-19, but how accurate are they?
Apple has been offering on-site employees a COVID-19 nasal swab test since May, but the company will also soon be shipping home test kits to remote staff.
The decision is part of Apple’s plan to have workers, including retail employees, continue working from home as new cases of COVID-19 in the US surge to record highs.
How one home collection kit works
COVID-19 home test kits enable individuals to collect nasal swab specimens on their own.
But the actual testing – to determine the presence of the SARS-CoV-2 pathogen – can take place only in certified laboratories that perform what the US Food and Drug Administration calls “high-complexity” tests.
One example of this product is the home collection kit developed by supermarket chain Kroger, which received emergency-use authorization from the FDA back in June.
Read more: COVID-19: How the world is caring for frontline workers
The home collection kit is “intended to facilitate self-collection” of nasal swab specimens, but the process should be supervised on video by a healthcare provider, the FDA said. The professional determines whether the steps are appropriate based on the results of a COVID-19 questionnaire.
“Once collected, the human specimen, which may include SARS-CoV-2 RNA, is maintained in the collection kit packaging during transport at ambient temperature to an authorized laboratory, designated by The Kroger Co., that runs the specimen on an authorized IVD molecular test for the detection of the virus that causes COVID-19,” the agency said.
Kroger’s proprietary tool is not the first home collection kit to get a nod from the FDA.
“Throughout this pandemic we have been facilitating test development to ensure patients’ access to accurate diagnostics, which includes supporting the development of reliable and accurate at-home sample collection options,” FDA Commissioner Dr. Stephen Hahn said.
“The FDA’s around-the-clock work since this outbreak began has resulted in the authorization of more than 50 diagnostic tests and engagement with over 350 test developers,” he said.
In Canada, researchers led by Professor John Brennan of McMaster University have also been working on a commercial home test kit that can reportedly yield results in as fast as 30 minutes.
It’s also pain- and hassle-free. Instead of undergoing a nasal swab test, the user would only have to expel mucus, which would then be tested using a test strip and a combination of chemicals.
Similar to the one used in a home pregnancy kit, the test strip with a small amount of the mucus would reveal one line if the sample is negative for SARS-CoV-2 and two if the sample is positive.
Another kit that aims to expedite the COVID-19 screening process is the RapiGen home test kit, which can reportedly detect antibodies against SARS-CoV-2 in about five to 10 minutes. It also relies on the appearance of a coloured line to determine whether the specimen contains antibodies.
“These test kits are revolutionary in expediting the process of testing since it cuts out the processing time by a fraction, aiding in an earlier detection of the virus, a more accurate prediction of the positive cases and a decrease of the infection rate,” said Marshall Gunter, CEO of DataMetrex, maker of RapiGen.
Read more: Which employees are exempt from returning to the worksite?
The risks of home testing
The Canadian government recognises that rapid test devices are often “simple to use and provide visual results within a short time period”. As such, they make it “possible for a greater number of people to be tested”.
“However, without the guidance of a healthcare professional, there is a significant risk that a patient could use the home test kit improperly or misinterpret the results,” officials said.
“At this time, Health Canada does not consider that the benefits of using home test (self-testing) kits outweigh the risks.”